Clinical Trial
STRO-001-BM1
Sutro is currently advancing two wholly owned clinical-stage programs designed to provide patient benefit across multiple areas of unmet need.
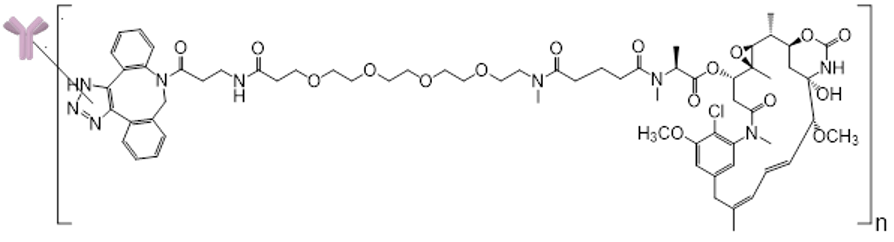
STRO-001-BM1 is a CD74 Targeting antibody drug conjugate (ADC) for the potential treatment of multiple myeloma and NHL.
- STRO-001-BCM1 is an ongoing first-in-human, phase 1, open-label, multicenter, dose escalation study evaluating the safety, tolerability and preliminary anti-tumor activity of STRO-001-BM1 in adults with B-cell malignancies (NHL and multiple myeloma).
STRO-001-BM1 is a novel homogeneous ADC using precisely positioned non-natural amino acids.
- STRO-001-BM1 targets the tumor cell carrying two cytotoxins, these are non-cleavable maytansinoid linker-warheads (DAR=2) that are stable in circulation
- The active warhead is internalized by the tumor cells, efficiently killing them while minimizing damage to surrounding healthy cells.
STRO–001-BM1 has received Orphan Drug designation for Multiple Myeloma patients.
For more information about this trial, visit www.clinicaltrials.gov
Sutro Biopharma
111 Oyster Point Blvd
South San Francisco, CA 94080
Phone: 650-881-6500
Fax: 650-553-9659
Contact Us
General Inquires: general@sutrobio.com
Business Inquires: busdev@sutrobio.com
Investor Relations Inquires: IR@sutrobio.com
Careers: jobs@sutrobio.com

