Clinical Trial
STRO-002-GM1
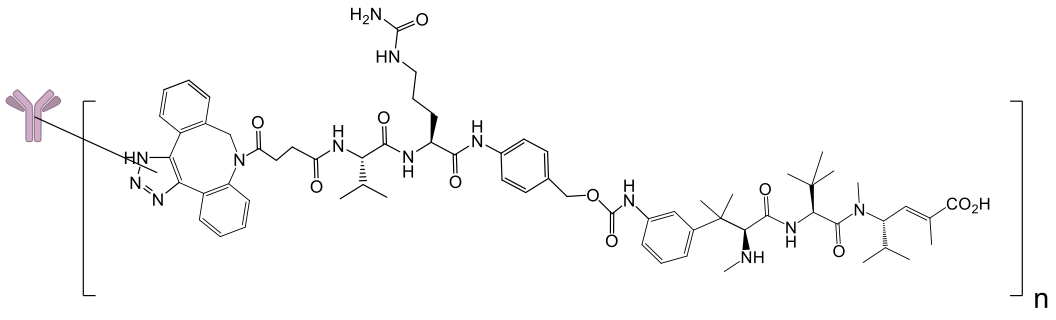
STRO-002-GM1 is a FolRa targeting ADC for the potential treatment of ovarian and endometrial cancer
- STRO-002-GM1 is currently in an ongoing first-in-human, phase 1, open-label, multicenter, dose expansion study evaluating the safety, tolerability and preliminary anti-tumor activity of STRO-002-GM1 in adults with ovarian cancer.
- STRO-002 was granted Fast Track designation by the U.S. Food and Drug Administration (FDA) in August, 2021 for the treatment of patients with platinum-resistant epithelial ovarian, fallopian tube, or primary peritoneal cancer who have received one to three prior lines of systemic therapy.
STRO-002-GM1 is a novel homogeneous antibody drug conjugate using precisely positioned non-natural amino acids
- STRO-002-GM1 targets the tumor cell carrying four cytotoxins, these are proprietary cleavable hemiasterlin linker-warheads (DAR=4) that are stable in circulation
- The active warhead is internalized by the tumor cell, efficiently killing them in a manner that can stimulate the immune system, an effect called immunogenic cell death.[1]
For more information about this trial, visit www.clinicaltrials.gov
[1]Source: June 22, 2020 AACR 2020 Virtual Meeting II – Abstract
Sutro Biopharma
111 Oyster Point Blvd
South San Francisco, CA 94080
Phone: 650-881-6500
Fax: 650-553-9659
Contact Us
General Inquires: general@sutrobio.com
Business Inquires: busdev@sutrobio.com
Investor Relations Inquires: IR@sutrobio.com
Careers: jobs@sutrobio.com

